Do you remember Zhu Fengcai, an SEU alumnus enrolled in 1987 and a part-time professor of SEU, who was once reported in the official WeChat. Results of the world’s first human trial of novel coronavirus vaccinewas published in The Lancet, the top international magazine. All trials generated the immune response! SEU alumnus Zhu Fengcai released the latest clinical trial results.

Recently, Zhu Fengcai’s team achieved new progress in the clinical trial of novel coronavirus vaccine. Let’s check it out.
As reported by the Xinhua News Agency on July 20, the Chinese research team published a paper in The Lancet, aBritish medical journalon the exact day, saying that they conducted the Phase 2 clinical trial of novel coronavirus vaccine, and the results showed that the vaccine was safe and could induce the human body to produce the immune response.
Researchers including Academician Chen Wei from the Institute of Bioengineering, the Academy of Military Medicine of the Chinese Academy of Military Sciences and Professor Zhu Fengcai from Jiangsu Provincial Center for Disease Control and Prevention conducted this clinical trial. The team had previously completed the Phase 1 clinical trial of the vaccine with related results also published.
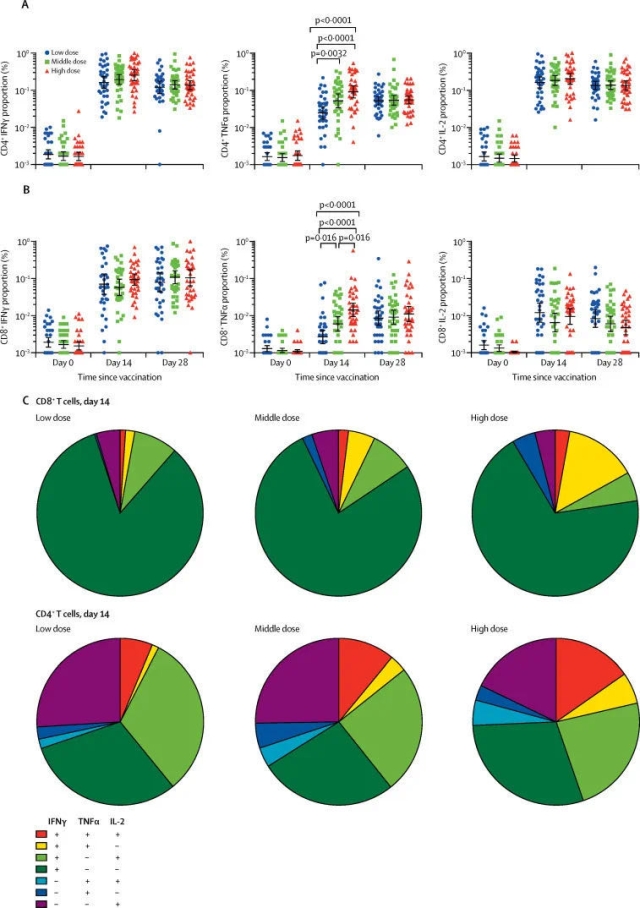
Related trials evaluated an adenovirus vector recombinant novel coronavirus vaccine. As introduced in the paper, more than 500 volunteers participated in the Phase 2 clinical trial in China, including people over 55 years old. The range of participants was even larger when compared with that in the Phase 1 clinical trial. The trail was to evaluate whether this vaccine could induce an immune response in the human body and whether it was sufficiently safe. The results showed that the vaccine had produced good results in both aspects.
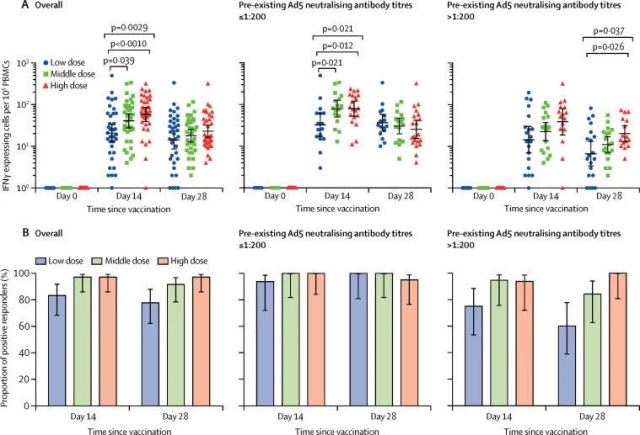
Zhu Fengcai said in a press release issued by The Lancet that the Phase 2 clinical trial, when compared with the Phase 1 clinical trial, has provided further evidences for the safety and immunogenicity of the vaccine in a larger population. This is an “important step” for evaluating the candidate vaccine. The team is currently conducting the Phase 3 clinical trial.
The researchers also pointed out that the volunteers engaged in the trial were not exposed to the novel coronavirus after being vaccinated. Therefore, we cannot judge whether the vaccine can effectively protect people from the novel coronavirus based on the trial results, which have to be further verified in the Phase 3 clinical trials.
Asintroduced in The Lancet, currently there are about 250 candidate novel coronavirus vaccines in the world with at least 17 of them in clinical trials.
















